What is CAPA and Why It’s Mandatory in Pharmaceuticals?
Written By :
Dr. Dhriti Tupe, GxP Expert ®
In the pharmaceutical industry, Corrective and Preventive Action (CAPA) is one of the most critical aspects of quality management. CAPA plays a pivotal role in maintaining product safety, efficacy, and compliance with regulatory standards. Let’s explore what CAPA is, why it’s mandatory, and why it’s crucial for the pharmaceutical industry.
Understanding CAPA
CAPA is a systematic approach used to identify, address, and prevent recurring issues that can affect the quality of a product. It consists of two main components:
Corrective Action: Actions taken to eliminate the root cause of a detected non-conformance or undesirable situation. In simple terms, it’s what you do to correct the problem once it has been identified.
Preventive Action: Proactive steps taken to eliminate potential causes of non-conformances before they occur. This is about prevention and making sure similar issues don’t arise in the future.
Together, CAPA ensures that issues are not only resolved but also prevented from reoccurring, which is vital in an industry where product quality impacts patient safety.
Why is CAPA Mandatory in Pharmaceuticals?
1. Regulatory Compliance
Pharmaceutical companies operate under strict regulations set by organizations such as the #FDA, #EMA, and #WHO. These regulatory bodies require firms to have a CAPA system in place as part of their quality management system (QMS). Failure to comply can result in fines, recalls, or even closure of facilities.
2. Ensuring Product Quality
CAPA is essential to ensure that pharmaceutical products are manufactured consistently and meet predefined quality standards. This is particularly important when deviations, non-conformances, or product failures are identified. CAPA helps companies correct the issues swiftly, ensuring the product is safe for consumer use.
3. Enhancing Patient Safety
In the pharmaceutical industry, errors or deviations can lead to significant risks to patient safety. CAPA helps to mitigate these risks by addressing the root causes of issues before they can affect consumers. By preventing the recurrence of quality-related problems, CAPA ensures that patients receive safe and effective products.
4. Preventing Financial Loss
Quality failures can result in costly product recalls, litigation, and reputational damage. A robust CAPA system helps companies avoid these financial repercussions by ensuring that problems are resolved before they become widespread. Preventive actions, in particular, can save companies from dealing with repeat issues, which is far more expensive in the long run.
5. Fostering Continuous Improvement
CAPA is not just about compliance—it’s a tool for continuous improvement. By investigating the root causes of issues and preventing their recurrence, CAPA drives a culture of improvement across the organization. This ensures that the company is always evolving and learning from its experiences to produce higher-quality products.
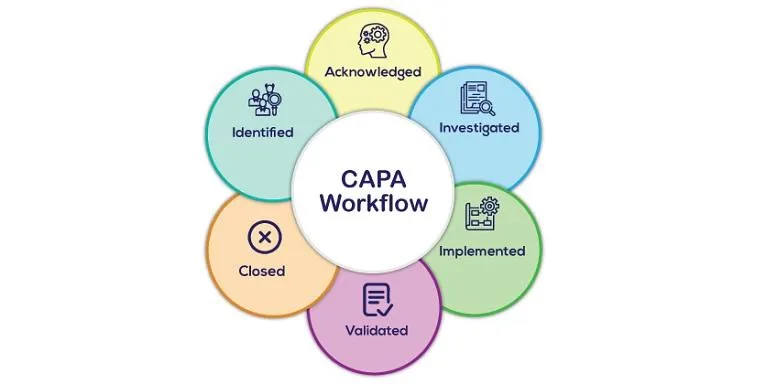
The CAPA Process: Key Steps
Implementing an effective CAPA system involves several key steps:
1. Identification: Recognising the non-conformance, deviation, or issue.
2. Investigation: Performing a thorough root cause analysis to understand why the issue occurred.
3. Corrective Action: Taking steps to resolve the issue.
4. Preventive Action: Implementing measures to prevent the issue from recurring.
5. Verification: Ensuring that the corrective and preventive actions taken have been effective.
6. Documentation: Recording all actions and outcomes to maintain transparency and traceability.
Summary
CAPA is more than just a regulatory requirement; it’s a cornerstone of quality assurance in the pharmaceutical industry. Its role in safeguarding product quality, ensuring patient safety, and fostering continuous improvement makes it mandatory. In a field where the stakes are so high, CAPA ensures that problems are not only fixed but also prevented, allowing pharmaceutical companies to maintain high standards of safety, compliance, and operational excellence.
For those working in quality assurance or quality management, mastering CAPA is essential for maintaining the trust of regulators, patients, and stakeholders alike.
Written By :
Dr. Dhriti Tupe, GxP Expert ®
Quality Compliance Lead Mentor Ph.D. MBA LLS- MB, GB, BB LSS-Minitab LSS Expert-Harvard Publishing Case Studies LLS-Healthcare CQA and IRCA Certified ISO 9001:2015 Lead Auditor GxP Consulting Adviser Pfizer