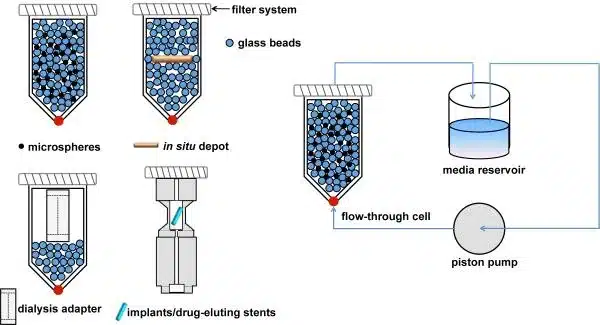
In Vitro Release Test (IVRT) for In Situ Gel/Depot-Forming Drug Products
Introduction:
In situ gel and depot-forming drug products have become a focus in the pharmaceutical industry for their ability to provide prolonged drug release at the administration site. This characteristic makes them ideal for treatments requiring sustained drug delivery, improving patient compliance and therapeutic outcomes. In this context, In Vitro Release Testing (IVRT) becomes essential to evaluate and ensure the effectiveness of such drug products before they enter clinical practice.
Challenges in IVRT Method Development:
Developing an IVRT method for in situ gel/depot-forming drug products is complex due to the unique formulation behaviors. Unlike traditional drug products, these formulations undergo physical transformations at the administration site, forming a gel or depot that gradually releases the drug.
Key challenges include:
– Absence of Compendial Methods:
Currently, there are no official compendial methods for IVRT of in situ gel/depot-forming products, leaving much of the development to exploratory and empirical approaches.
– Lack of Defined Study Conditions:
Product-specific guidelines often lack clear recommendations on the conditions necessary for an IVRT study.
– Discriminatory Ability:
Ensuring that the IVRT method can differentiate between slight variations in the formulation or manufacturing process that could impact drug release.
Understanding Gel/Depot Formation and Drug Release:
IVRT for in situ gel and depot-forming products must mimic the real-world scenario where the drug product transforms at the administration site.
There are two main mechanisms:
- Self-Aggregating Peptide Drugs: In this case, peptides self-aggregate upon administration, forming a dense fibrillar network. Drug release occurs as peptides diffuse from the network into surrounding tissues.
- Polymer-Based Formulations: These formulations involve the diffusion of organic solvents out of the drug and the precipitation of polymers, forming the depot. Drug release is influenced by factors like burst release, diffusion, and polymer degradation.
Key Parameters for IVRT Method Development:
Several critical parameters must be optimized for successful IVRT development:
– Sample Preparation: Ensure the drug sample forms a gel or depot before being loaded into the IVRT apparatus.
– Apparatus and Release Medium: Selecting the right apparatus and release medium is crucial to replicate the in vivo environment.
– Flow or Stirring Rate, Temperature, Sampling Time: These parameters help simulate physiological conditions and affect the drug release rate. It’s important to choose conditions that are physiologically relevant and allow for reproducible drug release profiles.
Gel/Depot Inducing Conditions:
When developing an IVRT method, special attention must be paid to the conditions that promote gel or depot formation. This includes investigating:
– Sample Amount and Media: The volume of drug sample and release media can impact the rate and extent of depot formation.
– Incubation Temperature and Time: These factors must simulate body conditions to allow for accurate evaluation of drug release.
IVRT Method Validation:
Once an IVRT method is developed, it needs validation. Key considerations include:
– Robustness: Ensuring that the IVRT method consistently provides reliable results across different batches and formulations.
– Discriminatory Ability: The method should distinguish between products that may fail to achieve bioequivalence (BE) due to variations in formulation, manufacturing, or depot formation.
Typical Deficiencies in IVRT Studies:
Several common issues arise in IVRT studies for in situ gel and depot-forming products:
– Incomplete Gel/Depot Formation: Inconsistent or poorly formed gels lead to high variability in drug release profiles.
– Inadequate Exploration of Conditions: Failure to thoroughly explore physiologically relevant gel/depot-inducing conditions can result in inaccurate conclusions regarding drug release behaviour.
Submission Content for ANDA:
When submitting an IVRT study as part of an Abbreviated New Drug Application (ANDA), the content must be thorough and well-documented:
– IVRT Method Development and Validation: Detailed reports of the IVRT method, including all study protocols and standard operating procedures (SOPs), are necessary.
– Raw Data: Complete raw data sets, including individual concentration datasets, must be provided to demonstrate the rate and extent of drug release.
Conclusion:
IVRT for in situ gel and depot-forming drug products is a critical tool for assessing the rate and extent of drug release in a controlled environment. The development and validation of an IVRT method must account for the unique challenges of these formulations, including the need for a well-formed and consistent gel or depot at the administration site. By addressing these challenges and adhering to rigorous validation protocols, IVRT can effectively support the development of bioequivalent generic drug products.
This approach helps manufacturers ensure product quality, safety, and efficacy before proceeding to clinical trials or market approval.