GLP-1 Single, Dual, and Triple Receptor Agonists for Treating Type 2 Diabetes and Obesity: A Comprehensive Narrative Review
Introduction
The global burden of obesity and type 2 diabetes mellitus (T2DM) continues to rise, affecting millions of individuals worldwide. Obesity and T2DM are intertwined, with obesity being a major risk factor for developing insulin resistance and T2DM, leading to serious complications such as cardiovascular disease and metabolic syndrome. Managing both conditions has become a focal point in healthcare, with incretin-based therapies, particularly glucagon-like peptide-1 receptor agonists (GLP-1RAs), emerging as pivotal treatment options. This review explores the efficacy, mechanisms, and development of single, dual, and triple receptor GLP-1 agonists, which are revolutionizing the treatment of T2DM and obesity.
Mechanism of Action of GLP-1RAs
GLP-1 is a peptide hormone secreted by the enteroendocrine L cells in the small intestine, particularly postprandially. Its main physiological actions include:
1. Enhancement of insulin secretion:
GLP-1 stimulates insulin release in response to glucose levels, particularly after meals, aiding in blood glucose control.
2. Inhibition of glucagon secretion:
GLP-1 reduces glucagon production from pancreatic α-cells, thereby lowering hepatic glucose output.
3. Slowing gastric emptying:
This results in a reduced postprandial glucose spike, contributing to overall glycemic control.
4. Appetite regulation:
GLP-1 acts on the central nervous system (CNS), particularly the hypothalamus and hindbrain, to suppress appetite and promote satiety, which leads to weight loss.
These actions make GLP-1RAs an effective treatment for both T2DM and obesity. However, in patients with T2DM, the incretin effect is diminished due to impaired GLP-1 signaling and increased insulin resistance. By mimicking or enhancing GLP-1 activity, GLP-1RAs help restore this lost functionality.
Single Receptor GLP-1 Agonists
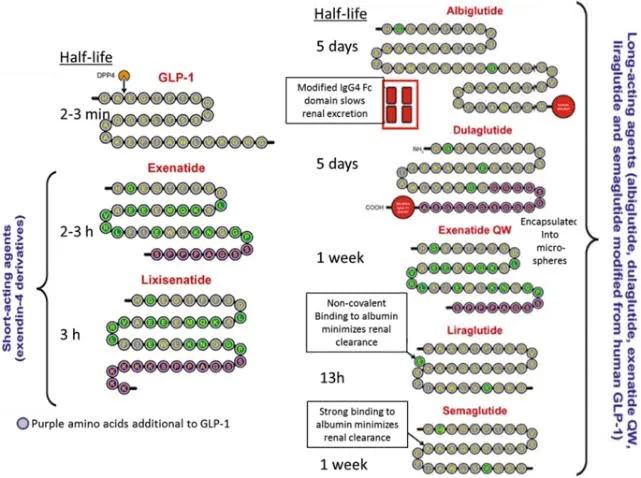
1. Exenatide:
Exenatide, a synthetic GLP-1 analog derived from the exendin-4 peptide found in the saliva of the Gila monster, was the first GLP-1RA approved for T2DM management. It is administered twice daily and was shown to reduce HbA1c by 0.8-1.1%. Exenatide also induces modest weight loss due to its effects on satiety and gastric emptying. A once-weekly formulation (Exenatide ER) was later developed to improve adherence and convenience.
2. Liraglutide:
Liraglutide, a human GLP-1 analog, has become a cornerstone of GLP-1 therapy for both diabetes and obesity. Administered as a once-daily subcutaneous injection, liraglutide offers significant benefits in lowering HbA1c (up to 1.5%) and promoting weight loss. Its ability to reduce cardiovascular events in T2DM patients led to its expanded use in obesity management under the brand name **Saxenda**. At a higher dose (3.0 mg), liraglutide has been shown to reduce body weight by 8-10% in clinical trials, making it a leading choice for patients struggling with both conditions.
3. Semaglutide:
Semaglutide is a second-generation GLP-1RA with a significantly longer half-life, allowing for once-weekly subcutaneous administration. Clinical trials (SUSTAIN and STEP programs) demonstrated that semaglutide is highly effective in lowering HbA1c (up to 1.8%) and promoting substantial weight loss (up to 15-17%). It is available in both injectable and oral formulations, making it a versatile option. Notably, semaglutide has shown superiority over other GLP-1RAs, such as exenatide and liraglutide, in terms of both glycemic control and weight reduction.
Dual Receptor GLP-1 Agonists
The development of dual receptor agonists, particularly those targeting GLP-1 and glucose-dependent insulinotropic polypeptide (GIP), represents a significant advancement in incretin therapy. These agents exploit the complementary actions of GLP-1 and GIP to achieve superior metabolic outcomes.
1. Tirzepatide:
Tirzepatide is a first-in-class dual GLP-1/GIP receptor agonist. It acts on both GLP-1 and GIP receptors, enhancing insulin secretion, reducing glucagon levels, and improving glycemic control. Tirzepatide also promotes weight loss by suppressing appetite and improving energy balance. Clinical trials (SURPASS program) showed that tirzepatide leads to HbA1c reductions of up to 2.1% and body weight reductions of up to 20% in patients with T2DM and obesity. Tirzepatide is administered once weekly and has demonstrated greater efficacy than single receptor GLP-1 agonists like semaglutide.
2. CagriSema (Semaglutide/Cagrilintide):
CagriSema is a combination of semaglutide and cagrilintide (an amylin analog). This combination enhances weight loss by promoting satiety and reducing food intake. In clinical trials, CagriSema demonstrated superior weight loss compared to semaglutide alone, with weight reductions exceeding 15%. It is currently under development for the treatment of T2DM and obesity.
Triple Receptor Agonists
The most recent innovation in incretin therapy is the development of triple receptor agonists, which target GLP-1, GIP, and glucagon receptors. These agents hold great promise in achieving unprecedented levels of glycemic control and weight loss by harnessing the unique benefits of each receptor pathway.
1. Retatrutide:
Retatrutide is a novel triple receptor agonist that targets GLP-1, GIP, and glucagon receptors. In phase 2 clinical trials (TRIUMPH), retatrutide demonstrated profound reductions in both HbA1c and body weight, with some patients achieving weight losses of up to 24%. By activating glucagon receptors, retatrutide also increases energy expenditure, contributing to its potent weight-loss effects. These results make retatrutide one of the most promising agents under investigation for both T2DM and obesity.
Safety and Side Effects
While GLP-1RAs are generally well-tolerated, the most common side effects are gastrointestinal in nature, including nausea, vomiting, and diarrhea. These side effects are usually dose-dependent and can be mitigated by gradual dose escalation. However, patients may experience increased heart rate and a small risk of pancreatitis. Long-term cardiovascular benefits have been well documented, with multiple GLP-1RAs showing reduced risks of major adverse cardiovascular events (MACEs), particularly in patients with high cardiovascular risk.
Dual and triple receptor agonists such as tirzepatide and retatrutide have shown a comparable safety profile to single receptor GLP-1RAs, though they may induce a slightly higher incidence of gastrointestinal side effects due to their potent weight-loss effects. Importantly, ongoing trials like the SURPASS and SELECT studies continue to monitor the long-term cardiovascular and renal safety of these novel agents.
Cost-Effectiveness
The cost-effectiveness of GLP-1RAs, particularly in the context of long-term management of T2DM and obesity, remains a topic of debate. While these agents offer substantial health benefits, their high cost may limit accessibility for some patients. Nevertheless, the reduction in long-term complications of diabetes and obesity, such as cardiovascular disease and renal impairment, may justify the investment in these therapies from a healthcare system perspective.
Conclusion
GLP-1 receptor agonists have revolutionized the management of type 2 diabetes and obesity, offering effective glycemic control and significant weight loss. The development of dual and triple receptor agonists represents the next frontier in incretin-based therapy, with agents like tirzepatide and retatrutide showing unprecedented efficacy. As research continues, these therapies are expected to play an increasingly important role in the long-term management of T2DM and obesity, improving patient outcomes and reducing the global burden of these chronic conditions. Further studies are needed to assess their long-term safety, cost-effectiveness, and potential applications in broader populations.