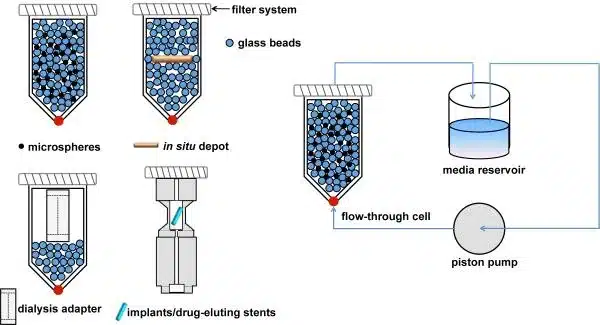
What Are Pharmaceutical Quality Control Laboratories and Why Are They Important?
What Are Pharmaceutical Quality Control Laboratories and Why Are They Important? By :Dr. Dhriti Tupe, GxP Expert ® In the pharmaceutical industry, Quality Control (QC) laboratories play a vital role in ensuring the safety, efficacy, and quality of pharmaceutical products. These laboratories are essential to maintaining the integrity of the drug manufacturing process, from raw materials…