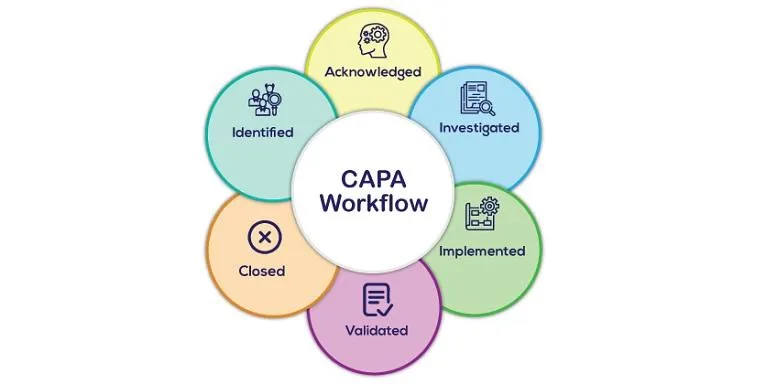
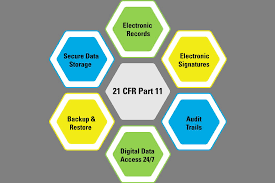
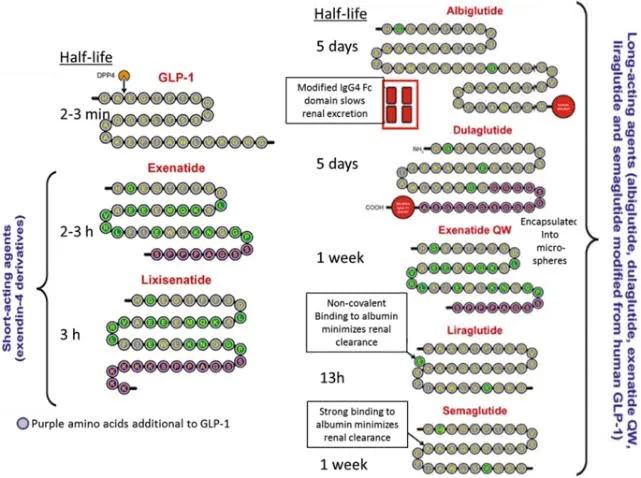
Do you review or Do you preview ?
Do you review or Do you preview ? By Sauri Gudlavalleti Picture this: You’re stuck in traffic, late for your engagement, anxiously staring at the Google Maps app on your phone – and the app is flooding you with copious amounts of information about how you got this far – average speed, number of turns,…