Understanding FDA’s Deficiency Response Letters for ANDA Submissions: A Guide to Successful Outcomes
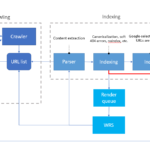
In the pharmaceutical industry, obtaining approval from the U.S. Food and Drug Administration (FDA) for an Abbreviated New Drug Application (ANDA) is a critical step toward bringing generic drugs to market. However, this process often involves receiving deficiency response letters from the FDA. These letters—Discipline Review Letters (DRLs), Information Requests (IRs), and Complete Response Letters…